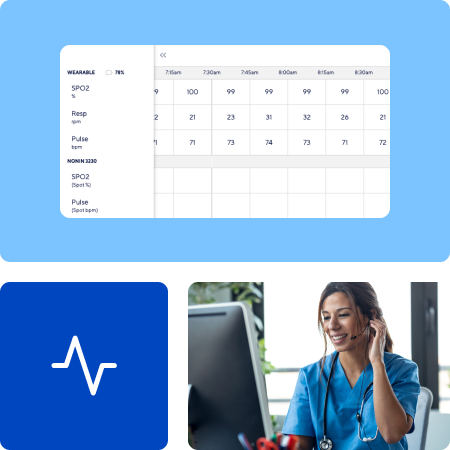
FDA-Cleared Continuous Monitoring Platform
Identify adverse events earlier with our ICU-levl continuous monitoring of heart rate, respiratory rate, SpO2, movement, and axillary temperature. Get intelligent alarms based on multiple data points and customize monitoring for each trial.